Radical addition to the vinyl C=C bond: Quantum chemistry model of the reaction
Thermochemical Aspect
Let us begin the analysis of radical addition from its thermochemical aspect. Although radical addition in general has a relatively low activation barrier and hence early TS, the possibility of relations between Properties of TSs and products were discussed long ago. An explanation is that due to spin polarization (which begins at early stages of the process) TS with an unpaired C=C bond resembles the product by its electronic structure.
Tables II and III list some reactions heats (ΔΔHf) and barriers (ΔΔH≠f). First, the evident conclusion is that β-attack is preferable in all cases considered, by 5 kcal/mol on average. The difference can be explained thermochemically (formation of a secondary vs. primary radical as a product) or sterically (less repulsion and so less deformation in TS). As Figure 1 shows, in all considered series of “radical → monomers” there exists a nearly linear correlation between reaction heat and activation energy (a kind of Polani–Semenov correlation). Points that correspond to β-addition (lower groups of points) make more certain plots.
So, we can state that the calculated heat of radical addition to C=C is an index of nearly the same power as the calculated gas-phase activation energy.
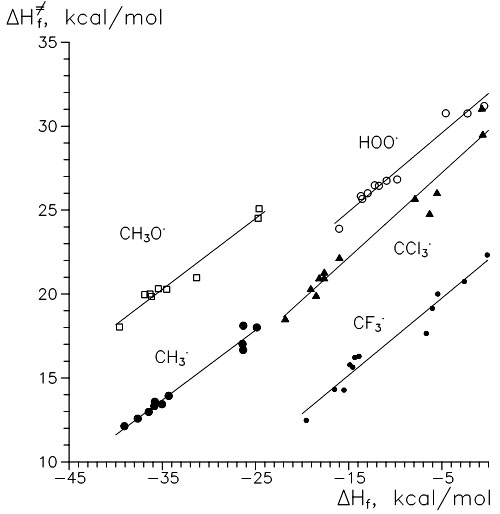 Figure 1. Relations between calculated heats of reactions and calculated activation barriers.
Figure 1. Relations between calculated heats of reactions and calculated activation barriers.
HOO–CH2–CHX• | HOO–CHX–CH2• | |||
|---|---|---|---|---|
| ΔΔHf | ΔΔH≠f | ΔΔHf | ΔΔH≠f | |
| Eth | −11.76 | 26.44 | ||
| Prop | −13.71 | 25.83 | −4.59 | 30.77 |
| St | −18.82 | 23.00 | — | — |
| AN | −12.97 | 26.01 | −2.26 | 30.76 |
| MA | −10.94 | 26.75 | −0.47 | 31.20 |
| VA | −16.03 | 23.89 | −9.81 | 26.83 |
| VCl | −12.21 | 26.48 | −9.87 | 30.15 |
| VCl2 | −13.55 | 25.67 | −5.98 | 32.03 |
CH3–CH2–CHX• | CH3–CHX–CH2• | |||
|---|---|---|---|---|
| ΔΔHf | ΔΔH≠f | ΔΔHf | ΔΔH≠f | |
| Eth | −34.32 | 13.93 | ||
| Prop | −35.80 | 13.58 | −26.32 | 18.12 |
| St | −41.68 | 11.44 | ||
| AN | −36.46 | 12.98 | −26.40 | 17.02 |
| MA | −35.04 | 13.44 | −24.86 | 18.01 |
| VA | −39.08 | 12.13 | −26.34 | 16.67 |
| VCl | −35.87 | 13.32 | −30.99 | 16.90 |
| VCl2 | −37.64 | 12.58 | −27.50 | 19.61 |
